research use only
Edaravone Antioxidant chemical
Cat.No.S1326
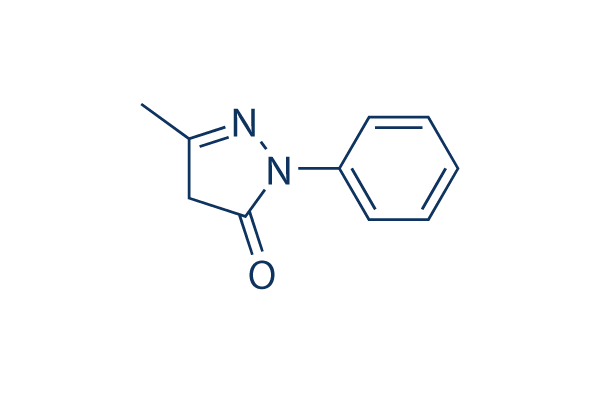
Chemical Structure
Molecular Weight: 174.2
Quality Control
| Related Targets | HSP JNK ROS eIF PERK IRE1 PKR ASK PDI ATF-6 |
|---|---|
| Other Antioxidant Inhibitors | Elamipretide (SS-31, MTP-131) (+)-Catechin Hydroxygenkwanin Silymarin (-)Epicatechin Syringic acid Cocoa Extract Oleuropein Clematis apiifolia Extract Parsley Extract |
Solubility
|
In vitro |
DMSO
: 35 mg/mL
(200.91 mM)
Ethanol : 4 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 174.2 | Formula | C10H10N2O |
Storage (From the date of receipt) | 3 years -20°C powder (seal) |
|---|---|---|---|---|---|
| CAS No. | 89-25-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | MCI-186 | Smiles | CC1=NN(C(=O)C1)C2=CC=CC=C2 | ||
Mechanism of Action
| In vitro |
Edaravone exerts neuroprotective effects by inhibiting endothelial injury and by ameliorating neuronal damage in brain ischemia. This compound provides the desirable features of NOS: it increases eNOS (beneficial NOS for rescuing ischemic stroke) and decreases nNOS and iNOS (detrimental NOS). This chemical, which inhibits oxidation and enhances NO production derived from increased eNOS expression, may improve and conserve cerebral blood flow without peroxynitrite generation during reperfusion.
|
|---|---|
| In vivo |
Edaravone significantly reduces the infarct volume and improves the neurological deficit scores at 24 hours after reperfusion in mice brain. This compound markedly suppresses the accumulation of HNE-modified protein and 8-OHdG at the penumbra area during the early period after reperfusion and reduces microglial activation, iNOS expression, and nitrotyrosine formation at the late period. It attenuates renal function and pathologic findings significantly in rat kidney. This chemical significantly reduces the generation of free radicals in the tubular cells indicated by dichlorodihydrofluorescein. Treated animals shows significantly improved neurological outcome. This treatment provides a significant reduction in the number of TUNEL-positive apoptotic cells, a decrease in Bax immunoreactivity and an increase in Bcl-2 expression within the peri-infarct area. It shows an excellent neuroprotective effect against ischemia/reperfusion brain injury through a Bax/Bcl-2 dependent anti-apoptotic mechanism.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06107205 | Completed | Healthy Adult Subjects |
Auzone Biological Technology Pty Ltd |
November 7 2023 | Phase 1 |
| NCT05953103 | Recruiting | Subjects With Cerebral Hemorrhage |
Simcere Pharmaceutical Co. Ltd |
July 3 2023 | Phase 2 |
| NCT05410457 | Recruiting | Ischemic Stroke |
Nanjing First Hospital Nanjing Medical University |
May 24 2022 | -- |
| NCT04776135 | Completed | Healthy Adult Subjects |
Mitsubishi Tanabe Pharma Corporation |
February 26 2021 | Phase 1 |
| NCT04254913 | Completed | Japanese Patients With ALS |
Mitsubishi Tanabe Pharma Corporation |
January 24 2020 | Phase 1 |
| NCT05342597 | Completed | Healthy Adult Subjects |
Mitsubishi Tanabe Pharma Corporation |
June 10 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































