research use only
Chlorpromazine HCl Dopamine Receptor inhibitor
Cat.No.S2456
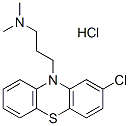
Chemical Structure
Molecular Weight: 355.33
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Histamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Dopamine Receptor Inhibitors | MPTP Hydrochloride Trifluoperazine Trifluoperazine 2HCl Penfluridol Sulpiride SCH-23390 hydrochloride SKF38393 HCl Levosulpiride Domperidone Rotundine |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| CHO cells | Function assay | Compound was evaluated for the binding affinity against [3H]8-OH-DPAT-labeled 5-hydroxytryptamine 1A receptor sites in cloned CHO cells, Ki=0.673 μM | ||||
| CHO cells | Function assay | Compound was evaluated for the binding affinity against [3H]U-86,170-labeled D2 sites in cloned CHO cells, Ki=0.003 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 71 mg/mL
(199.81 mM)
Water : 71 mg/mL Ethanol : 71 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 355.33 | Formula | C17H19ClN2S.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 69-09-0 | Download SDF | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
Dopamine receptor
Potassium channel
|
|---|---|
| In vitro |
Chlorpromazine affects miniature IPSCs (mIPSCs) by decreasing the binding (kon) and by increasing the unbinding (koff) rates of GABAARs. Chlorpromazine modulates activated TRPA1 currents in a voltage-dependent way, leading to a block at positive potentials and an increased open probability at negative potentials.
|
| In vivo |
Chlorpromazine independently down-regulates the production of various T cell-derived lymphokines (IL-2, IFN-gamma, IL-4, TNF, and GM-CSF) and up-regulates the secretion of IL-10 in an in vivo model of acute superantigen-driven immune activation. Chlorpromazine -mediated amplification of the SEB-driven Chlorpromazine secretion is accompanied by an enhanced IL-10 mRNA accumulation. Chlorpromazine protects mice, normal or adrenalectomized, and guinea pigs against lethality of LPS, and inhibits TNF serum levels. Chlorpromazine protects against LPS lethality when administered 30 minutes (min) before, simultaneously, or up to 10 min after LPS and is ineffective when given 30 min after LPS, paralleling the inhibitory effect on TNF production. Chlorpromazine significantly inhibits LPS lethality and hepatotoxicity in mice sensitized to LPS toxicity by actinomycin D, whereas under these conditions DEX is inactive. Chlorpromazine protects brain tissue from hypoxia-induced irreversible loss of synaptic transmission in rats. Chlorpromazine also significantly delays the occurrence of the hypoxia-induced spreading depression (SD) in rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06238089 | Recruiting | Intellectual Disability |
University of Plymouth|Peninsula Clinical Trials Unit |
December 19 2023 | -- |
| NCT05206747 | Recruiting | Mania|Sleep|Bipolar Disorder |
Ottawa Hospital Research Institute |
September 7 2022 | Not Applicable |
| NCT03548714 | Completed | Alcohol Dependence|Alcohol Interaction |
Pop Test Oncology LLC|Pharmacotherapies for Alcohol and Substance Use Disorders Alliance|Congressionally Directed Medical Research Programs|Michael E. DeBakey VA Medical Center|Baylor College of Medicine|University of California San Diego |
September 1 2018 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































