|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C53H84NO14P |
|||
| Molecular Weight | 990.21 | CAS No. | 572924-54-0 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (100.98 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Ridaforolimus (Deforolimus, MK-8669, AP23573) is a selective mTOR inhibitor with IC50 of 0.2 nM in HT-1080 cell line; while not classified as a prodrug, mTOR inhibition and FKBP12 binding is similar to rapamycin. Phase 3. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Treatment of HT-1080 cells with Deforolimus induces a dose-dependent inhibition of phosphorylation of both S6 and 4E-BP1, with IC50 of 0.2 nM and 5.6 nM, respectively, and leads to a decrease in cell size, an increase in the proportion of cells in the G1 phase of the cell cycle, and inhibition of glucose uptake. Deforolimus displays significant antiproliferative activity a broad panel of cell lines with EC50 of 0.2-2.3 nM. Deforolimus potently and selectively inhibits VEGF production in a dose-dependent manner. [1] Deforolimus treatment significantly induces growth suppression in human NSCLC cell lines with IC30 values of 2.45-8.83 nM, with the exception of H157 with IC30 of >20 nM. Deforolimus treatment (2.8-5.9 nM) significantly dephosphorylates p70S6KThr389 in A549, H1703 and H157 cells, except H1666 that may express a resistant variant of mTORC1, and causes increased phosphorylation of pAKTser473 and pAKTThr308 in A549 and H1703 cells. Deforolimus in combination with the MEK inhibitors, CI-1040 or PD0325901 exhibits dose-dependent synergism in lung cancer cell lines, which is associated with the suppression of proliferation rather than enhancement of cell death, involving the inhibition of ribosomal biogenesis by 40% within 24 hours and a decreased polysome/monosome ratio. [2] |
||||
| In vivo | Administration of Deforolimus exerts significant antitumor effects in mice bearing PC-3 (prostate), HCT-116 (colon), MCF7 (breast), PANC-1 (pancreas) or A549 (lung) xenografts in a dose-dependent manner, and inhibits mTOR signaling in in SK-LMS-1 xenograft model associated with inhibition of tumor growth. [1] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
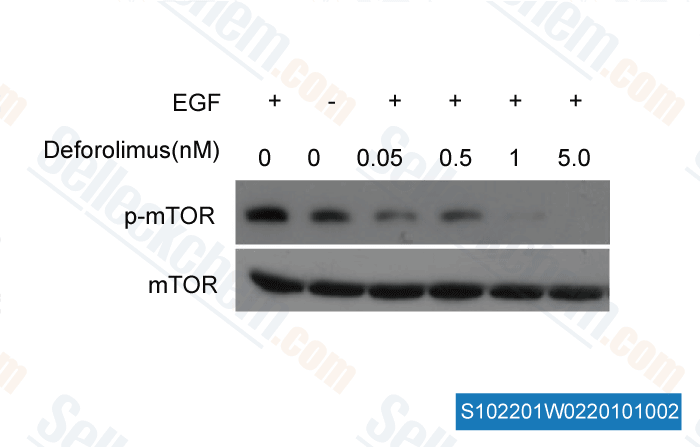
-
, , Dr. Zhang of Tianjin Medical University
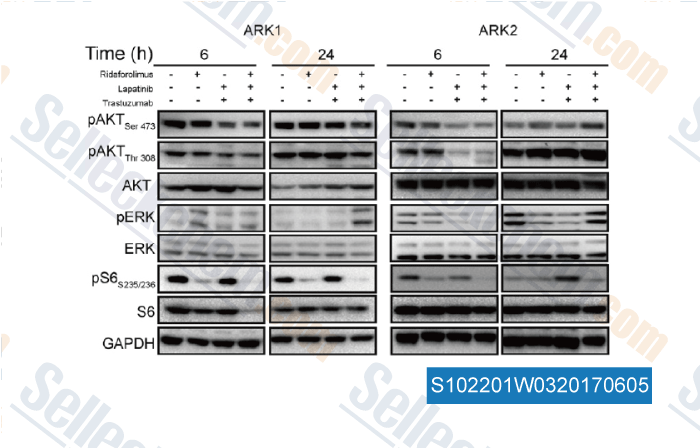
-
Data from [Data independently produced by , , Gynecol Oncol, 2016, 141(3):570-9]
Selleck's Ridaforolimus (Deforolimus, MK-8669) has been cited by 159 publications
| De novo generation of multi-target compounds using deep generative chemistry [ Nat Commun, 2024, 15(1):3636] | PubMed: 38710699 |
| TMAO enhances TNF-α mediated fibrosis and release of inflammatory mediators from renal fibroblasts [ Sci Rep, 2024, 14(1):9070] | PubMed: 38643262 |
| Identification and evaluation of a lipid-lowering small compound in preclinical models and in a Phase I trial [ Cell Metab, 2022, 34(5):667-680.e6] | PubMed: 35427476 |
| A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis [ Cell Metab, 2022, S1550-4131(22)00191-7] | PubMed: 35705079 |
| Structural identification of lysophosphatidylcholines as activating ligands for orphan receptor GPR119 [ Nat Struct Mol Biol, 2022, (9):863-870.] | PubMed: 35970999 |
| Molecular insights into biogenesis of glycosylphosphatidylinositol anchor proteins [ Nat Commun, 2022, 13(1):2617] | PubMed: 35551457 |
| Extracellular vesicles from lung tissue drive bone marrow neutrophil recruitment in inflammation [ J Extracell Vesicles, 2022, 11(5):e12223] | PubMed: 35595717 |
| Bladder Cancer-Derived Small Extracellular Vesicles Promote Tumor Angiogenesis by Inducing HBP-Related Metabolic Reprogramming and SerRS O-GlcNAcylation in Endothelial Cells [ Adv Sci (Weinh), 2022, e2202993] | PubMed: 36045101 |
| CRISPR/Cas9 Screens Reveal that Hexokinase 2 Enhances Cancer Stemness and Tumorigenicity by Activating the ACSL4-Fatty Acid β-Oxidation Pathway [ Adv Sci (Weinh), 2022, 9(21):e2105126] | PubMed: 35603967 |
| Global chromosome rearrangement induced by CRISPR-Cas9 reshapes the genome and transcriptome of human cells [ Nucleic Acids Res, 2022, 50(6):3456-3474] | PubMed: 35244719 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
